Monday, 31 August, 2009
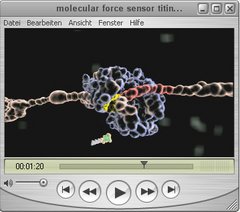
3D animation of the molecular force sensor titin kinase
Our muscles get stronger after excercise, but how do they sense where and when to grow? In combination with AFM-based single molecule force spectroscopy, molecular dynamics simulations and enzymatics we showed, that the autoinhibited muscle enzyme titin kinase gets activated by physiological forces (about 40 pN) and can act as a force sensor regulating gene expression and protein turnover.
The 3D animation shows a zoom inside the muscle up to the smalles protein building blocks to visualize how contraction is generated by myosin and actin filaments. In their center, these motor strand are connected and aligned through the elastic titin-network, which contains the molecular force sensor titin kinase. Force imbalances distort the elastic titin-network and open the binding pocked of titin kinase, which is now primed for docking of the cosubstrate ATP. This part of the animation shows molecular dynamics simulations performed at atomic resolution. Through the force-induced binding of ATP, titin kinase gets activated like a mechanical fuse and marks other reporter molecules with parts of ATP. The initiated signaling cascade regulates muscle gene expression and protein turnover.