Monday, 11 April, 2016
Blue boost for insulin secretion
Photopharmacology
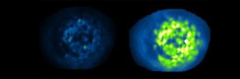
Islet cells in the pancreas infused with PhotoETP: Illumination with blue light (right panel) clearly induces activity. The image on the left shows the same islet prior to exposure to blue light. Source: D. Hodson
Researchers at LMU have equipped a signal receptor that plays an important role in the regulation of insulin secretion with a photosensitive switch. Exposure to blue light activates the switch and potentiates secretion of the hormone.
The incidence of Type 2 or adult-onset diabetes is rising rapidly worldwide. Diabetes is a chronic metabolic condition characterized by excess levels of glucose in the blood. The disease results either from reduced production of insulin by the pancreas or because the cells targeted by the hormone have become resistant to its effects.The receptor GLP-1R plays an important role in the regulation of insulin secretion, and therefore serves as a prime therapeutic target for the treatment of Type 2 diabetes. GLP-1R stimulates insulin secretion in response to binding of its natural ligand GLP-1. Now researchers led by Dirk Trauner, Professor of Chemical Biology and Genetics at LMU, in collaboration with David Hodson and his colleagues at Imperial College London, have developed a synthetic chemical that activates GLP-1R and augments insulin secretion when exposed to blue light. The new study appears in the latest issue of the journal “Angewandte Chemie”.
“Our new light-sensitive molecular switch interacts with a so-called allosteric site on the receptor, not with the orthosteric site to which GLP-1 binds,” says Johannes Broichhagen, first author of the new study. “The allosteric site is a region of the protein which is recognized by regulatory molecules. Binding at this site produces a structural alteration in the protein, which sensitizes the receptor by facilitating the binding of GLP-1.” Modulation of allosteric regulation can increase the specificity of drugs for receptors like GLP-R1 and open up opportunities for new therapeutic approaches. “This strategy has so far been hampered by the fact that the conformation of allosteric binding sites could not be controlled with sufficient precision,” says Trauner. The successful attachment of a light-sensitive molecular switch to a synthetic binding partner for its allosteric center solves this problem for GLP-1R.
The new compound, which its developers call PhotoETP, allows for exquisitely specific optical control of GLP-1R: “In its inactive form, PhotoETP binds to the receptor’s allosteric site. Exposure to blue light activates the switch, thereby inducing a conformational change in the receptor that potentiates insulin secretion. “This process can be carefully regulated, because the light can be very accurately delivered,” says Broichhagen. Of course, the effect is also dependent on binding of the ligand at the receptor’s orthosteric site. “There are basically two binding partners to be considered here, one of which normally activates GLP-1R relatively weakly,” says Broichhagen. “Strikingly, however, when we used PhotoETP and blue light to alter the conformation of the allosteric site, we found that binding of the weaker activator to the orthosteric site causes a twofold increase in insulin secretion.”
The researchers now plan to develop a variant of PhotoEP that can be activated by red light, which penetrates further into biological tissues. In addition, they will synthesize an array of structurally related molecules and test their effects on other receptors. “GLP-1R is a member of the large class of G-protein-coupled receptors, many of which are targeted by compounds of pharmaceutical and therapeutic interest,” says Trauner. “With PhotoETP, we now have a promising blueprint for the development of light-activatable molecules that are specific for other receptors of this type and could possibly be utilized as therapeutic agents. We can also use this approach to obtain a detailed picture of the mechanism that mediates cooperative interactions between the allosteric and orthosteric sites in GLP-1R and thus gain a better understanding of how the receptor works.“
Angewandte Chemie International Edition 2016


 Pressemitteilung LMU (Deutsch)
Pressemitteilung LMU (Deutsch)