Activities in our daily life are mainly limited by the turnover rate of biomolecular machines. After an extensive sport session, we need to regenerate our energy. Enzymes in our cells thereby resupply our stocks of molecules like ATP or ion pumps transport potassium and sodium ions back into our muscle cells. In order to do so, these biomolecular machines often undergo conformational changes. Even though the reaction path of many biomolecular machines is known, it becomes very challenging to study the switching speed between two conformational states, but quantification of dynamic processes is fundamental to understand many biological processes. Scientist study such dynamic processes and conformational changes with a highly sensitive spectroscopic ruler, the so-called Förster resonance energy transfer (FRET). Thereby, they read out the energy transfer between two organic dyes, which is distance dependent. The methode is so sensitive, that scientist can detect distance changes in single molecules with single FRET-pairs. For common fast fluctuations of the signal, scientist rely on the statistical analysis of many conformational changes of a single biomolecular machine. The analysis of the fluctuations is known as fluorescence correlation spectroscopy (FCS) but current realizations only yield the timescale of the conformational changes, not the forward and backward transition rate. Additionally, the interpretation of the intensity correlation can be ambiguous because the photophysics of the fluorescent dyes can result in artifacts. To rule them out, additional experiments are often required and result in an extensive amount of work.
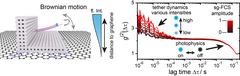
LMU scientists Dr. Tim Schröder, Prof. Don C. Lamb and Prof. Philip Tinnefeld and team now report an elegant approach to analyze intensity fluctuations by taking advantage of the excited state lifetime of the organic dyes. The change in fluorescence lifetime turned out to be the fingerprint of conformational changes of biomolecules in a FRET experiment. Disturbing photophysical processes do not change the fluorescence lifetime of the organic dyes. Thereby, the scientist filter the intensity regarding the fluorescence lifetime and only use subsets of the intensity signal for the intensity correlation. This enables to easily distinguish between photophysical artifacts from the organic dyes and to isolate the signal of interest of the biomolecule. "The new method, that we call shrinking gate (sg)-FCS, accelerates the analysis of intensity fluctuations significantly and yields information that was previously difficult to extract. We don't need prior assumptions or additional experiments and can focus on the interpretation of the data," says Dr. Tim Schröder, who led the development of the new technique.
The scientists demonstrated the power of this technique on well-defined model structures before applying the technique to other projects of the lab. The first application was the isolation of the Brownian motion of a biosensor on graphene. Binding of a protein slows this motion significantly down and a binding event is detected. In the second application, they unraveled the mechanism of a new FRET-based membrane-charge sensor, which they developed to study the membrane charge of cells. The charge sensor is anchored to the membrane and the scientist could show that the sensor works due to an equilibrium of temporal sticking to the membrane and detaching. This equilibrium is actuated by the surface charge of the membrane. "For more than 20 years FCS has been analyzed together with fluorescence lifetime but we still improve it today. That's exciting!" says Prof. Philip Tinnefeld. "Our new general approach offers a wide range for applications and we will work on exploring fast conformational changes in complex environment like living cells."


 Link to paper
Link to paper philip.tinnefeld
philip.tinnefeld