Monday, 07 October, 2019
Reactions on the edge
Catalysis

Model of an atomic step on a catalyst surface. At such sites on a cobalt catalyst, industrial Fischer-Tropsch synthesis of diesel fuel is discontinued. (Picture: J. Wintterlin / LMU)
Using the example of an important industrial process, CeNS member Prof. Joost Wintterlin shows that defects on the surface of catalysts are essential for their activity.
From chemical production to exhaust gas purification to the chemical storage of solar energy: many technical processes would not be possible without catalysts. In the chemical industry, the vast majority of products produced come into contact with at least one heterogeneous catalyst. Such catalysts are solid substances on whose surfaces gaseous substances adsorb and react. The catalyst enables or accelerates their reaction to give the product without changing itself. In this process, there are still many unanswered questions, such as where on the catalyst the process actually takes place. Chemical scientists around Professor Joost Wintterlin of the Department of Chemistry at LMU show that steps on the catalyst surface play a crucial role. They report about their results in the journal Nature Catalysis.
In many heterogeneously catalyzed reactions, there is indirect evidence that not the entire catalyst surface is active, but only areas with defects, such as the corners and edges of the catalyst particles, and not the smooth surfaces in between. "However, it has not yet been possible to show directly whether these areas are really the active centers, because it is very difficult to analyze the chemical processes on the surface under reaction conditions, i.e., at gas pressures of several bar and at elevated temperatures," says Wintterlin.
Wintterlin and his team have been working for some time on the development of a special scanning tunneling microscope with which catalytic reactions on surfaces can be examined under conditions close to those applied in industry. Instead of the catalyst particles, which are often only a few nanometers in size, the scientists use crystals that are several millimeters in size. In the work now published, the scientists also determined the formation of the catalytic reaction products on the same sample under the same conditions. "This is the only way to detect correlations between the structural elements of the surface shown under the microscope and the catalytic activity," says Wintterlin. "This combination makes the experiment particularly difficult." A specially developed gas chromatograph, with which extremely low product concentrations can be detected, finally led to success.
As an example for their analysis, the scientists chose the Fischer-Tropsch synthesis, a large-scale process in which liquid hydrocarbons such as synthetic diesel are produced from synthesis gas on a cobalt catalyst. For this system, the scientists were able to show that the catalytic activity of the sample increased the more atomic steps there were on the surface of the cobalt crystal used as the catalyst. The steps are caused by the fact that the atomic layers of the crystal on the surface are incomplete. At the point where one layer ends, a step to the next layer is created. Such steps also exist on the surfaces of the small cobalt particles of the industrial catalyst, and its activity could be quantitatively predicted with the data of the model catalyst. "This is the first direct proof that these atomic steps are the active centers of the catalyst," says Wintterlin. The scientists hope that these results could contribute to the development of more effective catalysts.
Nature Catalysis 2019
Source: 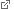 Press release LMU (in German)
Press release LMU (in German)

