Tuesday, 27 October, 2015
Dangling the bait
Photoswitches
CeNS researchers have developed a novel light-activatable chemical switch for transmembrane receptors that is highly stable under physiological conditions, and could potentially function in living organisms.
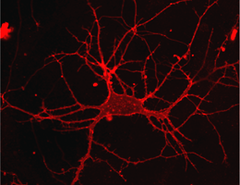
Highly specific cell-surface proteins are crucially involved in the transmission of signals between nerve cells. When triggered by the binding of an appropriate molecular ligand, these transmembrane receptors act as conduits to allow specific cations or anions to pass through the cell membrane, initiating a change in the electrical potential that is propagated to functionally linked neurons. “Hence the ability to control the function of these proteins by means of light provides a powerful tool for the investigation of neuronal function and is of potentially enormous significance in pharmacological research,” says Dirk Trauner, Professor of Chemical Biology and Genetics at LMU and a specialist in the development of optically responsive molecular switches. Trauner and his colleagues now describe a novel and versatile photoswitch for transmembrane receptors, which significantly extends the range of applicability of the technology. Their latest findings appear in the journal “ACS Central Science”, the newly launched interdisciplinary Open Access journal of the American Chemical Society.
A more stable adaptor
The properties and behavior of light can be controlled, both temporally and spatially, with exquisite precision. Consequently, proteins that have been equipped with a chemical switch whose conformation can be altered by light can be activated and deactivated with high spatial and temporal specificity. “However, the methods so far employed for the chemical attachment of photoactivatable switches to transmembrane proteins suffer from certain limitations,” says Dr. Johannes Broichhagen, first author on the new study. “First of all, there is the risk of undesirable cross-reactions, and secondly, the types of switches used cannot be introduced directly into the cell, and therefore cannot be used in vivo, because the functional group required to attach them to the target protein is unstable in living organisms.”
The LMU team therefore decided to try out a new approach to the labeling of proteins, which is based on the so-called SNAP-tag technology. In this case, the target protein of interest is fused to a small DNA repair enzyme called AGT - the eponymous SNAP-tag - using molecular genetic techniques. A cell-permeable so-called benzylguanine is then added, and reacts with the SNAP-tag enzyme to form a covalently bound adaptor. This in turn serves as an anchor for the subsequent attachment of a long linker to which the ligand itself is connected by a chemical bond. “Unlike the types of adaptor molecule employed in the photoswitches demonstrated so far, the benzylguanine is stable within the cell and in living organisms, so that this photoswitch can be used for a much wider range of applications,” says Broichhagen.
A switch with a long reach
In the conventional strategy, the linker contains a chemical bond whose orientation can be altered by irradiating it with light of a specific wavelength. This bond acts as a joint that can be extended to enable the attached ligand to activate the target protein or retracted to terminate activation. In the SNAP-tag approach, the bond that undergoes ‘photoisomerization’ is directly adjacent to the ligand – not located within the linker connecting it to the adaptor. “This means that the linker can be long and flexible. The switching molecule is tethered to it like the hook at the end of a fishing line, so that it can interact with a distant binding site in the target segment of the fusion protein. And this is important because the SNAP-tag itself is relatively large,” says Broichhagen.
As an initial test of the new strategy, Trauner and his group designed a photoresponsive ligand that binds to the transmembrane receptor mGluR2, which is involved in controlling the responsivity of nerve cells. “mGluR2 belongs to the large class of so-called G-protein-coupled receptors (GPCRs), and the majority of pharmacological agents interact with members of this family. So our new system could be of great therapeutic relevance,” says Trauner. “As a result of its simplicity and modular nature, the new system can easily be applied to many other proteins. It therefore offers a powerful tool with which to dissect molecular mechanisms and characterize neuronal networks with the aid of light.”
ACS Central Science 2015


 Press release LMU (German)
Press release LMU (German)