Friday, 23 October, 2015
A tail with alternate ending
Biophysics
CeNS scientists at the LMU Biophysics Department introduced a new tool for nanospectroscopy, a monovalent variant of Streptavidin, and thus revealed a surprising asymmetry of the energy landscape of the Strep-tagII:Strep-Tactin complex.
Investigated by a group of scientists around Dr. Diana Pippig and Prof. Hermann Gaub of the Applied Physics Department at LMU, a variant of the most prominently used non-covalent coupling agent (Strept)avidin, which recognizes and tightly binds a short peptide (Strep-tag II), was observed to exhibit a markedly asymmetric binding potential. The forces required to pull the peptide out of the binding pocket of Strep-Tactin via the N-terminal end were found to be twice as high as via the C-terminus. This drastically shows the importance of the elaborate arrangement of the constituents of force bearing or force regulating molecular complexes in cells or tissue. The finding also convincingly demonstrates the necessity of controlling the alignment and pulling geometry of the molecular components in in vitro investigations such as single-molecule force spectroscopy.
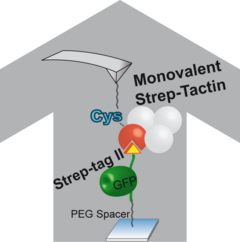
In an effort to establish a general, well-defined and robust tethering strategy to probe any protein of interest a monovalent Strep-Tactin was implemented. The tetramer harbored a single functional binding site for either Biotin or Strep-tag II, the latter is genetically encoded and can thus be easily fused to any protein of interest without the necessity of post-expression modification. A unique Cysteine residue opposite the ligand binding site was introduced to facilitate covalent coupling of monovalent Strep-Tactin to either functionalized surfaces, such as AFM cantilevers, or to for example fluorescent dyes. For AFM studies this defined geometry ensured a distinct force propagation pathway and thus allowed to identify the difference in unbinding forces when the Strep-tag II peptide was fused to either N- or C-terminus of a protein.
As a proof of principle two proteins with characteristic unfolding fingerprints, GFP and Titin Kinase, were tethered and pulled apart with the newly introduced handhold system. It could further be shown that owing to the monovalency of the system the cantilever wear-out is minimized and thus data yields are extremely high.
Monovalent Strep-Tactin can be utilized in mechanically stressed coupling reactions and for detection purposes. It can thus be considered a general tool in nanospectroscopy especially were one on one stoichiometries are desirable.
Baumann et al., Nature Nanotechnoloy