Monday, 16 August, 2010
Indications of a naturally occurring Diels-Alder reaction
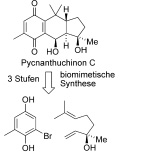
Otto Paul Hermann Diels and Kurt Alder were awarded the Nobel Prize in Chemistry in 1950 for their discovery of the reaction that now bears their names. Sixty years later, the Diels-Alder belongs to the classical reactions in organic (carbon-based) chemistry, but it has lost little of its fascination. The reaction provides a way to link two carbon compounds, a conjugated diene and a substituted alkene, to form a 6-membered hexene ring. This type of ring is the starting point for the production of an enormous variety of synthetic chemicals and natural products. Curiously, it is not entirely clear whether the biosynthesis of naturally occurring cyclohexenes actually involves the Diels-Alder reaction or employs one of the many other conceivable routes. Professor Dirk Trauners team at the Faculty of Chemistry and Pharmacology at LMU Munich and the Center for integrated Protein Science Munich (CiPSM), has just published a study which suggests that organisms may well make use of the Diels-Alder reaction. We have chemically synthesized a relatively complex natural product known as pycnanthuquinone C with the help of a Diels-Alder reaction under biological conditions, reports Trauner. The reaction cascade that we used is remarkably short, efficient and elegant. It seems almost inconceivable that the cells that produce pycnanthoquinone C make it in any other way. The natural version was originally isolated from a brown alga, and belongs to a group of compounds that are currently being tested as possible therapeutics for the treatment of type-2 diabetes. (suwe) (Angewandte Chemie, 16 August 2010)
Presseinformation der LMU (deutsch)
Press information LMU (english)
Publication "Biomimetische Synthese von (-)-Pycnanthuchinon C über eine Vinylchinon-Diels-Alder-Reaktion"